Catalyst for Proton Exchange Membrane Water Electrolysis with Low Iridium Loading developed
Erlangen, 12. Juli 2024 - Green hydrogen is a promising energy carrier that can bridge the gap between the supply and demand of renewable energies. Proton exchange membrane water electrolysis (PEMWE) is one of the most promising technologies to produce hydrogen. However, its large-scale implementation is currently delayed due to the utilization of iridium as the anodic catalyst which is extraordinarily scarce and expensive.
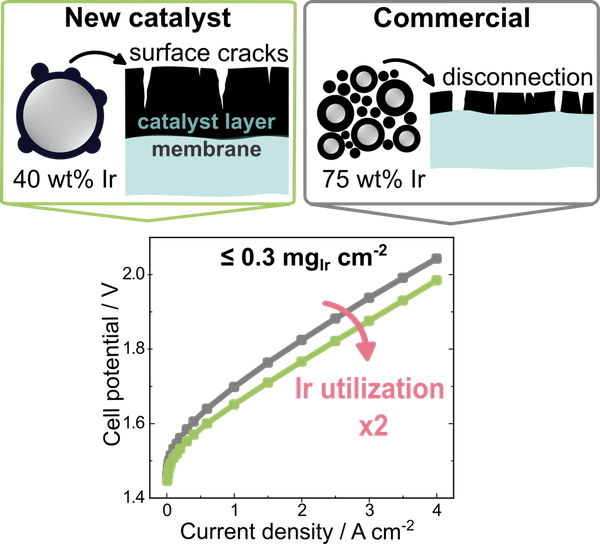
Researchers at the Helmholtz Institute Erlangen-Nuremberg for Renewable Energy (HI ERN) have now developed a new catalyst that allows a reduction in the amount of iridium required in a PEMWE cell. To achieve this, a facile photodeposition-based synthesis method for a TiO2@IrOx core-shell electrocatalyst with a low iridium content of 40 wt% was developed, and its superior performance in a proton exchange membrane water electrolyzer (PEMWE) was demonstrated. Owing to the low iridium content, this novel catalyst is specifically suited for reducing the iridium loading below 0.5 mgIr cm‑2 while maintaining a sufficient catalyst layer thickness.
The new catalyst doubles the iridium utilization, thereby reducing the amount of iridium needed in a PEMWE cell.
Their work was recently published in the renowned journal "Advanced Science".
Original Publication
Photodeposition-Based Synthesis of TiO2@IrOx Core–Shell Catalyst for Proton Exchange Membrane Water Electrolysis with Low Iridium Loading
https://onlinelibrary.wiley.com/doi/10.1002/advs.202402991
Journal: Advanced Science
License: CC BY 4.0
Contact
Dr.-Ing. Andreas Hutzler
Team leader "Nanoanalysis of Electrochemical Processes"
Room 4009
Dr. Chuyen Pham
Team leader "Catalyst Synthesis"
Room 0224




